- myLubrizol
-
-
Search Pharmaceuticals
For over six decades, the pharmaceutical industry has utilized multifunctional Carbopol® polymers for developing differentiated drug product innovations. Today, the Carbopol® polymer range continues to provide highly effective controlled-release properties for oral tablets at low usage levels, critical rheological and bioadhesive properties for topical formulations, and patient-centric benefits across multiple dosage forms, among many other advantageous properties.
With over half a century of technical expertise and extensive regulatory support, LLS Health is the ideal partner to support innovation from early formulation through commercial launch.

Carbopol® polymers help address market needs by:
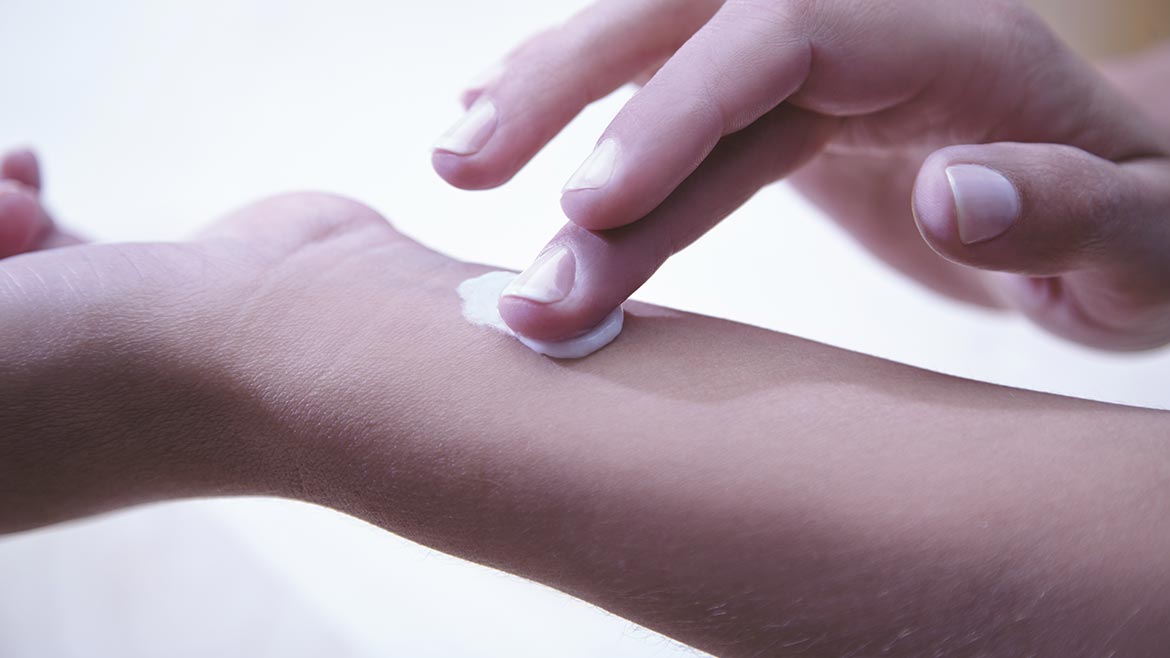
Carbopol® polymers provides critical qualities to topical treatments, including:
Samples can be requested at any time on our Contact Us page. Most of our sampling is handled by distributors, so you can either contact the distributor for your region directly or reach out to us and we can direct your request.
For preclinical trials only, Lubrizol excipient samples can be utilized. For full clinical trials, only fully GMP-compliant materials should be used. Lubrizol excipient samples are not typically GMP compliant and therefore should not be used for clinical trials, however larger sample quantities of GMP materials are available for clinical trials upon request. Non-sample quantities of our excipients are GMP compliant and can be used for clinical trials and in commercial products as well.
All relevant documents can be found in our Resource Hub. Please contact us if you require further assistance with technical information and resources If you cannot find what you are looking for.
No. Our polymers are chemically stable under normal storage conditions; there were no significant changes in the chemical parameters or detected impurities for a period of minimum 5 years. The physical stability related to maintaining less than 2% moisture level requires a Relative Retest Date of 2 years which can be tested by the customer using the Loss on Drying (LOD) procedure.
Our polymers should be stored in airtight containers protected from moisture and excessive temperature or temperature fluctuations.
Lubrizol has published two guides to help formulators with all their processing needs pertaining to Carbopol®, Pemulen™, and Noveon® polymers, including our Excipient Formulation and Processing Guide for Liquids and Semisolids and our Carbopol® Formulation and Processing Guide for Oral Solid Dosage Forms. If you require further assistance beyond these guides, Lubrizol offers extensive Technical Support for all dosage forms. Please contact us with your questions.
Carbopol® polymers are suitable to increase the viscosity of non-aqueous (anhydrous) vehicles such as propylene glycol, polyethylene glycol (PEG), glycerin, and alcohols. Low usage levels with efficient viscosity increase make Carbopol® polymers the ideal excipients for thickening anhydrous solvent systems. Additionally, no neutralization is required to provide thickening and suspending properties as well as clarity. You can learn more in our technical paper on the topic or by contacting us.
Every formulation is unique and there is no “one size fits all” approach when it comes to suitable excipients. However, our Abbreviated Excipient Guide is a reasonable starting point for determining what will work for your dosage form and specifications. For any additional support needed, our Technical Service group is available to assist you. Please contact us.
Lubrizol works with all relevant regulatory bodies to establish and maintain the global pharmacopeial status of its pharmaceutical ingredients. Lubrizol’s pharmaceutical grade carbomers are listed in monographs of the following international compendia:
Additionally, Lubrizol supports its pharmaceutical ingredients (Carbopol®, Pemulen™, and Noveon® polymers) with Drug Master Files (DMFs) in the United States and China. Regulatory excipient dossiers may be available to support drug applications in Europe and other regions. Visit our Regulatory page to learn more.
Lubrizol previously supplied some benzene-grade carbomers but has transitioned in recent years due to evolving global regulations. Lubrizol does not recommend the use of a benzene polymerized carbomers in a pharmaceutical formulation. The following table shows recommended substitutes for the benzene grade Carbopol® polymers based on viscosity criteria. The substitute products are polymerized in either ethyl acetate or a cosolvent mixture of ethyl acetate and cyclohexane. If a substitution is made in a pharmaceutical formulation, it is recommended that key performance properties be ascertained and regulatory considerations be taken into account. Depending on the desired dosage requirements, other Carbopol® polymers may be suitable alternatives.
| Benzene Grade Carbopol Polymer | Recommended Non-Benzene Carbopol® or Pemulen™ Polymers |
|---|---|
| Carbopol 934 NF polymer | Carbopol 5984 EP and Ultrez 10 NF polymers |
| Carbopol 934P NF polymer | Carbopol 974P NF polymer |
| Carbopol 940 NF polymer | Carbopol 980 NF and Ultrez 10 NF polymers |
| Carbopol 941 NF polymer | Carbopol 71G NF, 971P NF and 981 NF polymers |
| Carbopol 1342 NF polymer | Pemulen TR-1 and TR-2 NF polymers |
Both PC grade and pharma grade polymers are manufactured in cGMP facilities made to the highest industry standards. Pharmaceutical grade polymers require additional quality control and testing to the pharmaceutical monograph requirements including areas such as heavy metals and lower residual solvent testing.