Oral drug administration is critical for the delivery of drugs in various therapeutic areas, such as cardiovascular and pain management. It is considered the preferred route of administration due to its significant advantages, including being both painless and convenient. The importance of oral drug delivery is demonstrated by the fact that it comprises more than 50% of marketed pharmaceutical products, greater than any other single dosage form, and is growing at a compounded annual growth rate of around 6% for the coming years.
However, with such a large market, products need a way to differentiate themselves while still meeting increasing patient needs. We sat down with Rajib Bhattacharjee, Lubrizol Life Science Health’s (LLS Health) Global Market Manager for Oral Treatments, to discuss the importance of oral drug delivery and distinguishing your product in a crowded market, as well as innovation in the space of poorly water-soluble compounds, particularly amorphous solid dispersions.
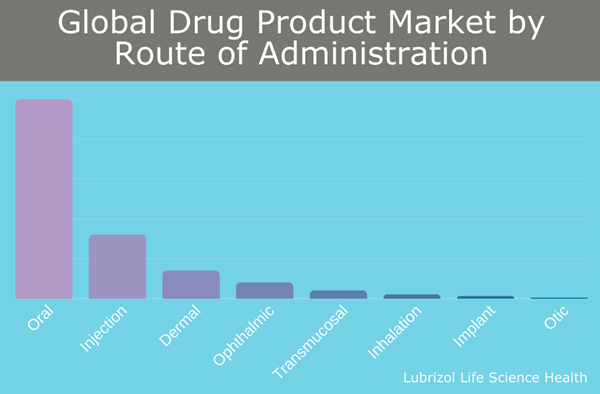
Figure 1: Global Drug Product Market by Route of Administration
What is oral drug delivery and what are some advantages of oral administration?
Oral drug delivery refers to methods, technologies, formulations, and systems used to transport a drug safely into the body via the oral cavity to achieve the desired therapeutic effect.
“Oral delivery is often the favored route of drug administration,” says Rajib. “Besides being available in various formats, including tablets, capsules, powders, and liquids, oral dosage forms offer other significant benefits to manufacturers and patients, such as being:
- Non-invasive, painless, and easy to administer,
- Often less costly and associated with fewer side effects when compared to some other routes of administration, such as injectables,
- Convenient for repeated and prolonged use, if needed,
- Able to be self-administered, without the need for physician assistance,
- Relatively simple to produce with minimal sterility constraints,
- Flexible in dosage form design.”
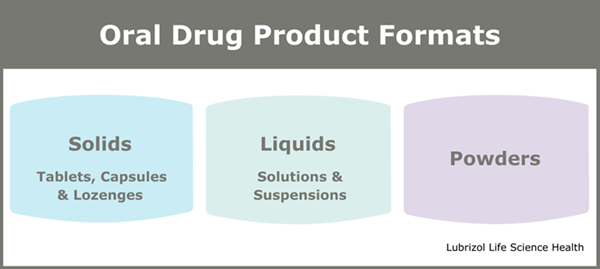
Figure 2: Types of Oral Drug Product Formats
What does the oral drug delivery space look like?
“Oral drug delivery is here to stay,” notes Rajib. “Oral drug delivery systems may not be as glamorous or cutting-edge as other areas of the pharmaceutical industry, but they still comprise a large portion of the existing pharmaceutical market, and of drug approvals each year.”
Currently estimated at several hundred billion dollars, the oral drug product market remains strong and is estimated to continue to grow at a healthy rate. While prescription (Rx) products, meaning those that require the permission of a medical professional to purchase, still encompass a significant portion of oral sales, over-the-counter (OTC) products have been gaining popularity since the 1990s. The OTC surge is driven by several factors, such as the increasing misuse and addiction awareness of prescription painkillers.
With such a large oral drug product market, how do you differentiate your product?
“The first step to differentiating your product is to first intimately understand the needs of the end user: the patient,” comments Rajib. “Improving patient outcomes should be central to any differentiation strategy. This can often be achieved via strategic excipient selection to impart patient-centric properties to a formulation.”

Figure 3: Examples of Patient Needs in Oral Treatments
“But we must not forget about the manufacturer as well. Making their jobs a little easier is always a plus,” Rajib says. “When designing an oral treatment, understanding formulation and manufacturing trends is also key when creating a truly stand-out product. Differentiation can take the form of increasing efficiency and processing ease.”
Take, for example, oral tablet manufacturing. Though traditional processes such as roller compaction and wet granulation remain popular, emerging technologies like fluid bed processing have found traction due to improved productivity from the eliminated need for granulation. Therefore, designing a product that can be processed via methods such as this, which require multifunctional excipients that are directly compressible, can be essential to distinguishing your product. Hot melt extrusion and spray drying are also becoming prominent, as they are the primary methods used to prepare oral systems that suffer from poor bioavailability, an increasingly common attribute.
What are some trends in oral treatments, especially around improving bioavailability?
“Around 70-80% of new chemical entities suffer from poor water solubility and subsequently poor bioavailability,” notes Rajib. “Therefore, a large portion of innovation occurring in oral drug delivery revolves around improving solubility.”
Many oral drugs have been made more soluble using various techniques and are now commercially available. Some of the methods used to increase solubility and bioavailability include:
- Particle size reduction (i.e, micronization or nanomilling)
- Self-emulsifying systems
- Salt or co-crystal formation
- Amorphous solid dispersions (ASDs)
ASDs, in particular, have emerged as one of the preferred methods to increase aqueous solubility of drugs. ASDs, on a very basic level, consist of an amorphous active ingredient stabilized by a polymer. Amorphous compounds are those that lack a uniform structure or order, the opposite of that seen in the original crystalline drug compound, and this lack of order makes it more readily available to the body. However, amorphous compounds are inherently unstable, hence the need for a stabilizing polymer that can offer both solubility enhancement and flexibility in the drug release profile.
ASDs offer substantial benefits, such as:
- The ability to be formulated into oral solids, whereas other solubility enhancement methods can only formulate oral liquids
- Higher capability for drug loading, which can reduce the number of oral tablets required for each dosage
With all these trends and the need for differentiation in oral products, where do you see the industry heading?
There is still significant unmet need in the oral drug delivery space and the industry is expected to see resultant growth. With the change in lifestyle leading to select disease prevalence, coupled with increased product demand from emerging economies, the oral sector needs innovative products and formats now more than ever.
“The industry will need to look towards groundbreaking methods for increasing bioavailability, as well as multifunctional ingredients that impart patient-centric properties to their drug product, if they want to achieve distinction in a large market,” says Rajib. “Success is very possible, however, especially if you partner with a sustainable organization driven by consumer and formulator needs and/or utilize an experienced CDMO. Pharmaceutical companies with an in-depth understanding of their patients, robust R&D capabilities, and strong partnerships will emerge victorious in the battle for differentiation and thus so will the end consumer, i.e. patients.”
LLS Health is a sustainable partner for all phases of drug product development. We are consumer and formulator-driven, working to enable innovations with the goal of improving patient quality of life. We enable formulators to meet the growing oral drug delivery demands through integrated excipient functionality, contract development and manufacturing services, and continuous technical and regulatory support.
Rajib Bhattacharjee | Global Market Manager, Oral Treatments
Ashley M. Rein | Technical Marketing Specialist