When handling plastics in the processing of materials, it’s important to note that not all plastics react the same way to fire. A common misconception is that of plastic’s tendency to melt under high temperatures, to produce toxic byproducts and flaming droplets, rendering it dangerous and unfit for purpose.
TempRite® Engineered Materials have developed CPVC to allow product developers to engineer for safety, with strong defences against fire. CPVC resins, and therefore many subsequent compounds are not only resistant to fire; they are chemically incapable of sustaining it.
In addition to this, product engineers can expect the following benefits from TempRite CPVC resins:
- Higher flash ignition softening temperature
- Better flame and smoke properties
- Lower smoke density
- Better flame retardancy
- Low thermal conductivity
Combustion of Ordinary Materials vs CPVC
TempRite CPVC is resistant to both fire and smoke production at the molecular level. While there is a reaction between fire and CPVC (and all burning produces smoke to a degree), its natural reaction is not to break down, rather, to protect.
The ordinary chain of combustion in ordinary materials happens as follows:
- The oxygen around the material is heated up and forms singlet oxygen
- Singlet oxygen searches for a reaction
- Hydrogen atoms are broken away forming free radicals
- Free radicals become active both in the material and in the air above the material
- Material’s molecular chain becomes unstable, volatile particles rise up
- Volatiles react with free radicals in the air to produce fire
- Combustion of volatiles also becomes smoke
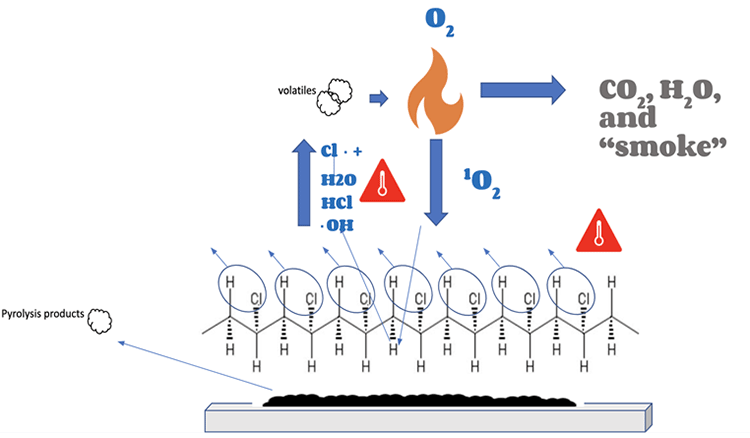
Alternatively, the combustion cycle of PVC looks like this:
- As PVC is heated, hydrochloric acid is knocked away from polymer and becomes gas (this is known as (zipper dehydrochlorination)
- Hydrochloric acid gas reacts with the free radicals, forming chlorine radicals and water. (These radicals are much less reactive and they do not contribute to fire.)
- Conjugated dienes are formed in the polymer, allowing PVC molecules to crosslink with each other
- The breakdown of PVC molecules is inhibited as the crosslinking creates a stable insulative char on the surface.
- Pyrolysis products are released as smoke, however this is much less so.
Combustion Cycle of PVC
TempRite CPVC is a highly chlorinated polymer. The following changes occur in CPVC’s combustion cycle:
Like PVC, zipper dehydrochlorination occurs, however this is inhibited due to the additional chlorine atoms in the chain. The molecular structure of the polymer therefore remains more stable.
This slower process promotes crosslinking from within the polymer, restricting the formation of volatiles even more, forming char faster.
Limiting Oxygen Index
TempRite CPVC’s ability to burn under ordinary atmospheric conditions is very limited. It has a very high Limiting Oxygen Index (LOI) of 60, meaning there would have to be 60% oxygen in the air for CPVC to burn. Ordinary PVC has an LOI of 45.
Earth’s atmosphere contains 21% oxygen. The only way Lubrizol CPVC can burn at normal atmospheric conditions is if an external flame is constantly applied. Furthermore, burning will cease as soon as the flame is removed.
It is important to be aware of the polymers that exist have LOI values below the atmospheric threshold and are therefore much more susceptible to burning:
Material Comparison: Limiting Oxygen Index
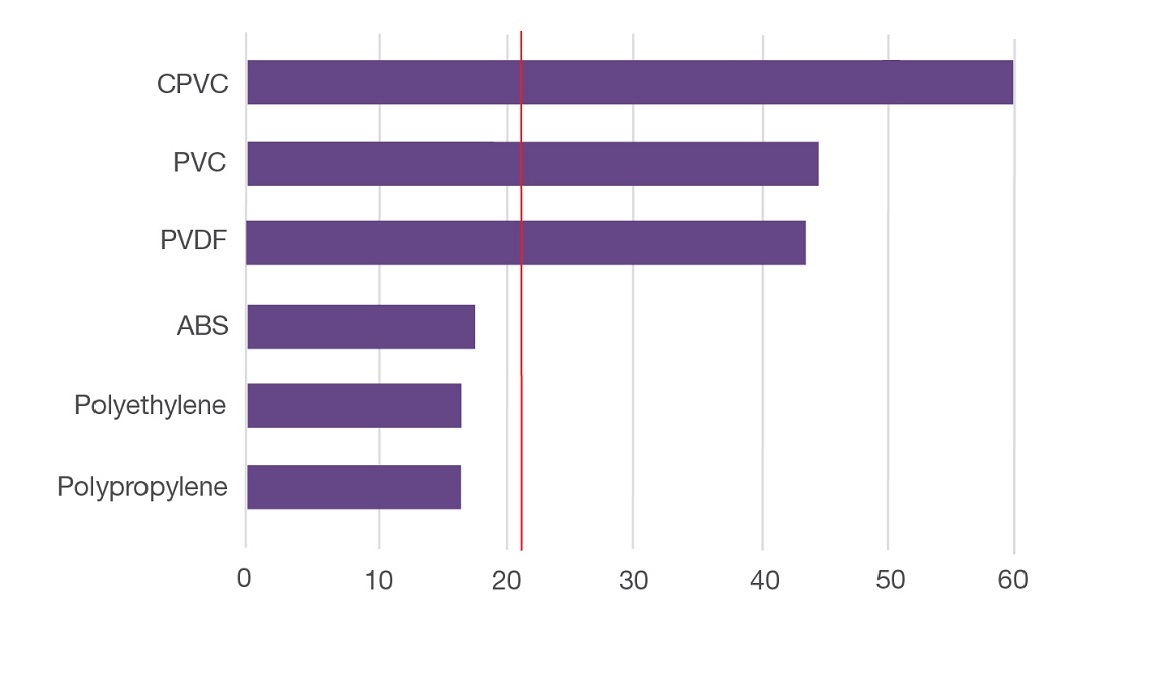
Materials below red line are susceptible to combustion under normal atmospheric conditions (21% oxygen)
Flash Ignition Temperature
A powerful indicator of a material’s resistance to fire is its Flash Ignition Temperature; the moment enough volatiles are reacting with free radicals to cause a fire (Flash ignition is when things finally catch fire.) This is the moment a material is hot enough to cause the material to break down and release volatiles into the air.
In the case of CPVC, Flash Ignition Temperature is very different from its rivals:
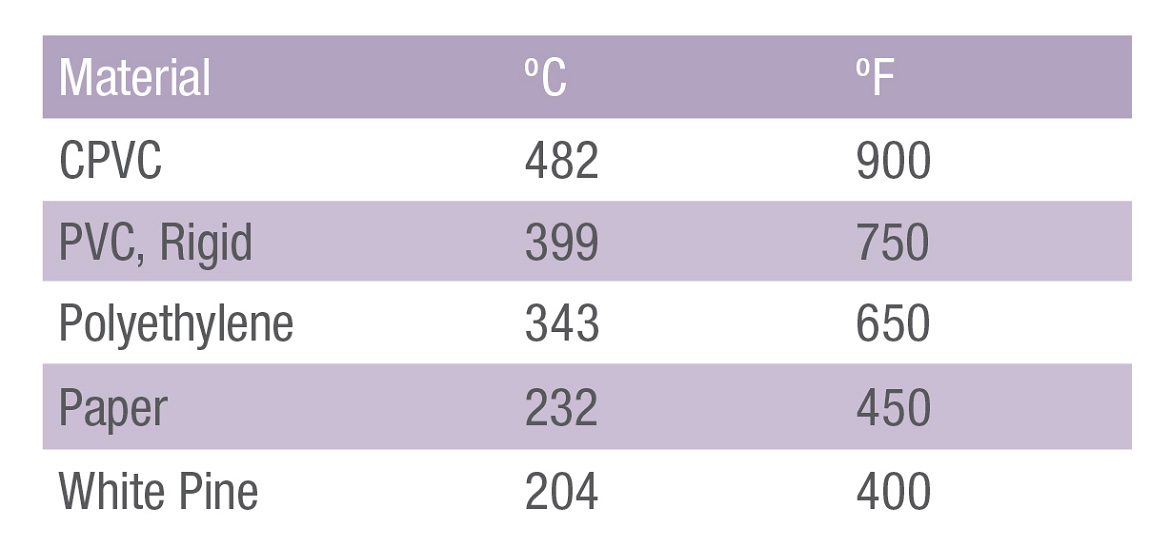
What Happens When CPVC is Exposed to Fire
One of the principal dangers of polymers when exposed to flames is the formation of burning drips. These can spread a fire from one area to another.
TempRite CPVC, when exposed to fire forms a charred layer, thanks to the rapid crosslinking process taking place from within the polymer. This barrier does not break away from the material; it spreads to create a layer of protection, maintaining the integrity of the material within.

If CPVC is exposed to fire, combustion does happen, however it protects itself during the process without producing burning drips, and it self-extinguishes when the source of the fire is removed.
This adds a new layer of protection to the development of fire protection materials, or the manufacture of products intended for use in hazardous environments.
CPVC and Smoke Toxicity
A question around plastics and combustion is what dangerous byproducts are produced when they burn.
TempRite CPVC’s smoke propagation is naturally low due to its molecular structure. The irregular chlorine structure limits the zipper dehydrochlorination process, making it slower. It forms a stronger char barrier and produces less volatiles, producing less smoke as a result.
The slower dehydrochlorination process of CPVC, resulting in lower smoke propagation
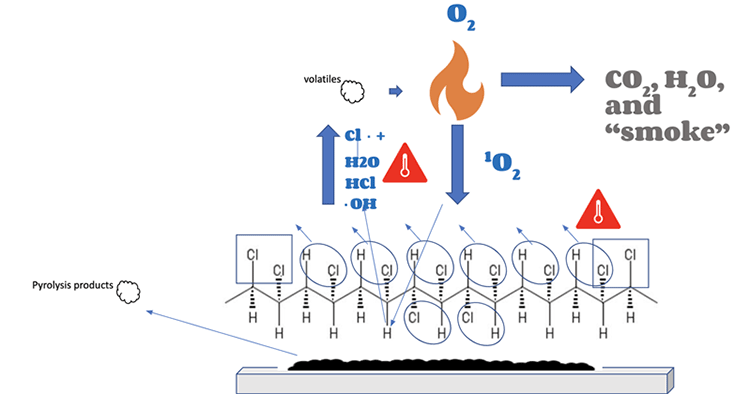
Pyrolysis products are released, as in all combustion, but not as dangerously as typical smoke.
All smoke is toxic to a degree; even products that “burn cleaner” (such as a domestic scented candle) release pyrolysis products into the atmosphere. CPVC’s very limited smoke production is another advantage for product designers who work in hazardous environments.
Applying CPVC’s Fire Resistance in Manufacturing
TempRite CPVC’s natural resistance against the development, spread and toxicity of fire provides exciting new opportunities in product development:
- Construction - wood effect plastic decking/lumber
- Cladding - exterior decorative paneling (foamed, lightweight)
- Plastic housing - electrical appliances
- Vinyl siding - wood effect plastic for residential construction in a variety of colours


